Plattenspieler trans-Orbitale: Unterschied zwischen den Versionen
KKeine Bearbeitungszusammenfassung |
|||
| (4 dazwischenliegende Versionen desselben Benutzers werden nicht angezeigt) | |||
| Zeile 1: | Zeile 1: | ||
{{:Theoretische Untersuchungen zu den Plattenspieler-Molekülen}} | |||
== Struktur == | == Struktur == | ||
| Zeile 9: | Zeile 11: | ||
== Orbitale == | == Orbitale == | ||
=== | === STS-Spektrum === | ||
[[Image:Plattenspieler_trans_DOS.png]] | <!-- [[Image:Plattenspieler_trans_DOS.png]] --> | ||
[[Image:STS-Spektrum_Plattenspieler.png]] | [[Image:STS-Spektrum_Plattenspieler.png]] | ||
| Zeile 56: | Zeile 58: | ||
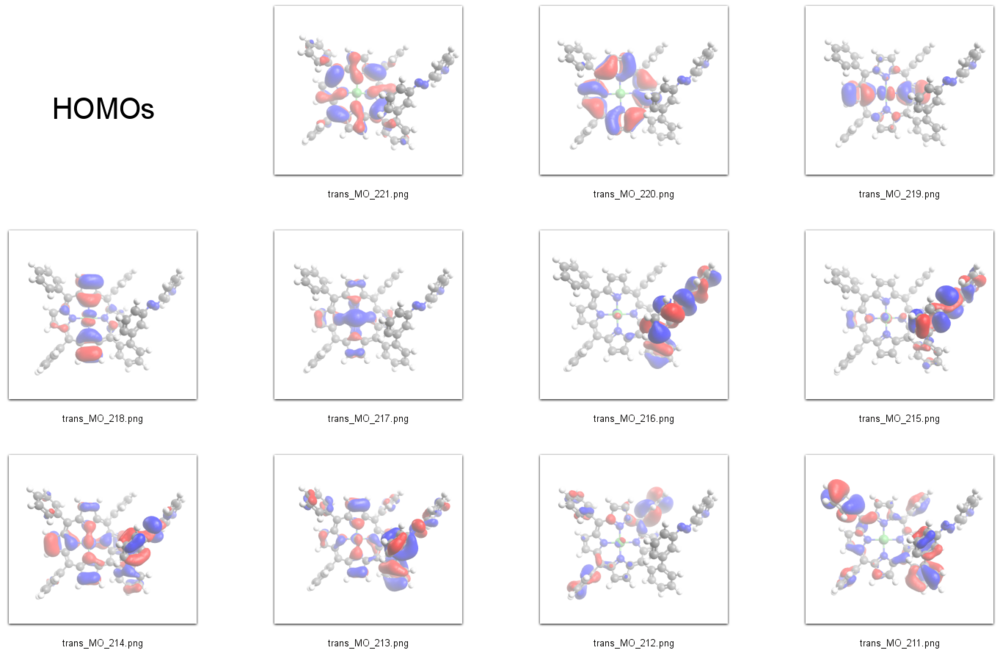
We therefore identify the signal at -1 eV in the STS spectrum of the ''cis'' isomer as the lone pair orbital mainly located at the azo-group of the azopyridine unit (MO=219). The azo group of the ''cis'' isomer is the highest part of the molecule with respect to the surface and its lone pair orbitals are pointing away from the surface and thus are exposed to the STM tip which gives rise to a large signal. | We therefore identify the signal at -1 eV in the STS spectrum of the ''cis'' isomer as the lone pair orbital mainly located at the azo-group of the azopyridine unit (MO=219). The azo group of the ''cis'' isomer is the highest part of the molecule with respect to the surface and its lone pair orbitals are pointing away from the surface and thus are exposed to the STM tip which gives rise to a large signal. | ||
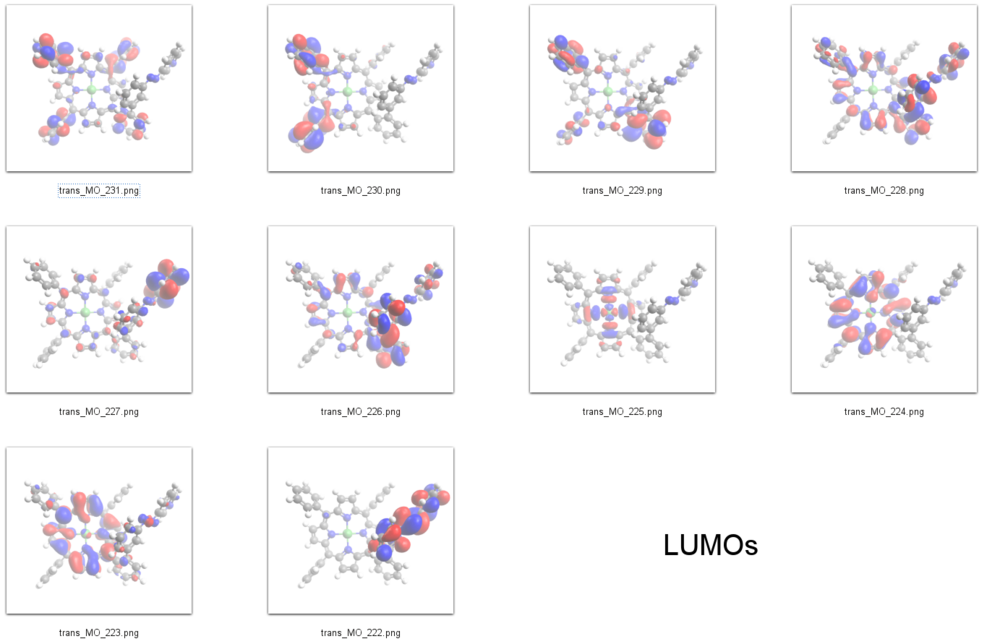
The peak at about -1.7 eV in the spectrum of the trans isomer and at -1.6 eV in the cis isomer can be assigned to the 𝜋<sup>*</sup> orbitals of the azopyridine units, which are the LUMOs of the | The peak at about -1.7 eV in the spectrum of the ''trans'' isomer and at -1.6 eV in the ''cis'' isomer can be assigned to the 𝜋<sup>*</sup> orbitals of the azopyridine units, which are the LUMOs in our DFT calculations (E<sub>cis</sub>=-2.58 eV(MO=222)), (E<sub>trans</sub>=-2.85 eV(MO=222)). | ||
The lower energy of the ''trans'' LUMO as compared to the ''cis'' thus is also in agreement with the STS spectrum. | |||
Aktuelle Version vom 22. Dezember 2009, 14:41 Uhr
Struktur
Orbitale
STS-Spektrum
LUMOs
HOMOs
Orbitalenergien
231 L+9 -0.65 A 230 L+8 -0.7 A 229 L+7 -0.74 A 228 L+6 -0.98 A 227 L+5 -1.15 A 226 L+4 -1.17 A 225 L+3 -1.5 A 224 L+2 -2.46 A 223 L+1 -2.49 A 222 LUMO -2.85 A 221 HOMO -5.33 A 220 H-1 -5.46 A 219 H-2 -6.16 A 218 H-3 -6.19 A 217 H-4 -6.39 A 216 H-5 -6.53 A 215 H-6 -6.76 A 214 H-7 -6.79 A 213 H-8 -6.87 A 212 H-9 -6.96 A 211 H-10 -6.99 A
Download
Dateien als Zip-Archiv runterladen
Interpretation of STS-spectra
According to our DFT-calculations (B3LYP/6-311+G*) the n and 𝜋 orbitals of the diazapyridine unit differ in the cis and trans configuration, similar to the parent azobenzene. The highest occupied orbital with coefficients located at the azopyridine unit (n type orbital) is considerably higher in energy for the cis as compared to the trans isomer (Ecis=-6.19 eV(MO=219)), (Etrans=-6.53 eV(MO=216)).
We therefore identify the signal at -1 eV in the STS spectrum of the cis isomer as the lone pair orbital mainly located at the azo-group of the azopyridine unit (MO=219). The azo group of the cis isomer is the highest part of the molecule with respect to the surface and its lone pair orbitals are pointing away from the surface and thus are exposed to the STM tip which gives rise to a large signal.
The peak at about -1.7 eV in the spectrum of the trans isomer and at -1.6 eV in the cis isomer can be assigned to the 𝜋* orbitals of the azopyridine units, which are the LUMOs in our DFT calculations (Ecis=-2.58 eV(MO=222)), (Etrans=-2.85 eV(MO=222)).
The lower energy of the trans LUMO as compared to the cis thus is also in agreement with the STS spectrum.