Plattenspieler trans-Orbitale: Unterschied zwischen den Versionen
Zur Navigation springen
Zur Suche springen
| Zeile 49: | Zeile 49: | ||
[http://134.245.135.211/download/trans_MOs.zip Dateien als Zip-Archiv runterladen] | [http://134.245.135.211/download/trans_MOs.zip Dateien als Zip-Archiv runterladen] | ||
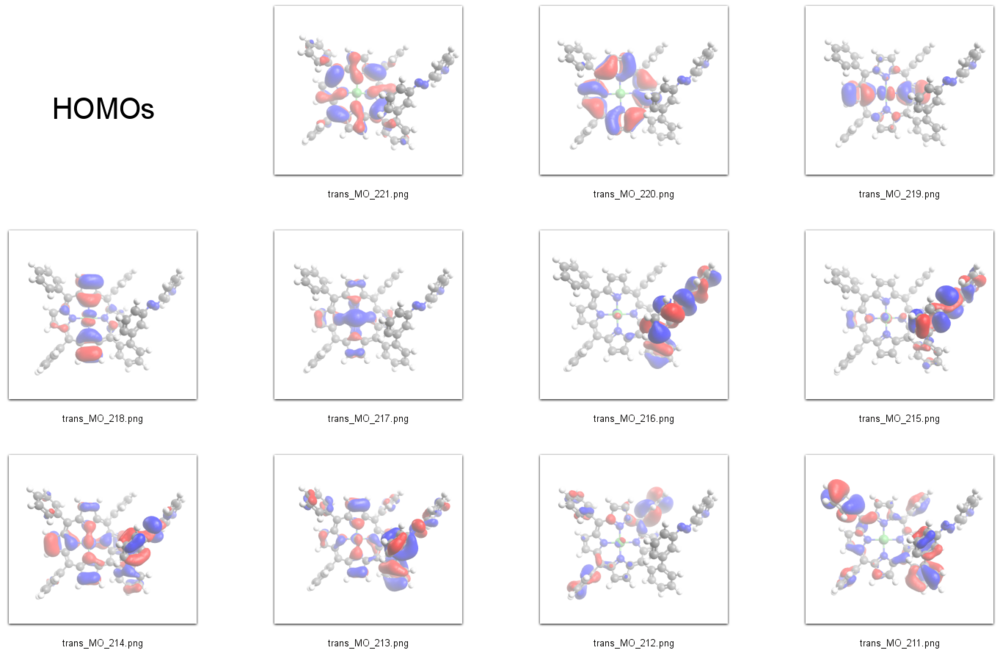
=== Interpretation of STS-spectra === | |||
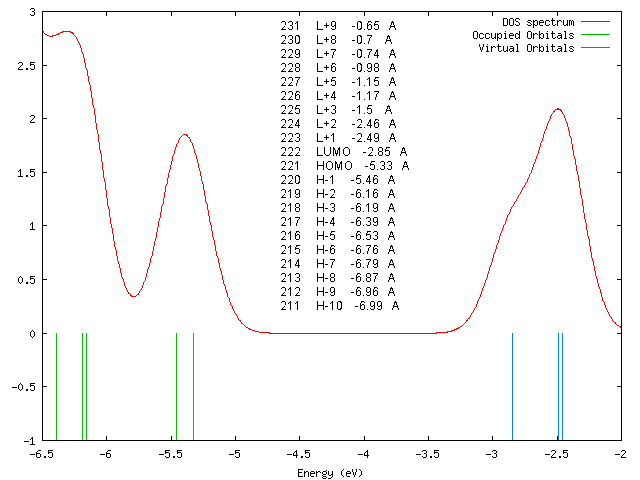
According to our DFT-calculations (B3LYP/6-311+G*) the Pi-N-Orbitals of the diazapyridine unit differ in the ''cis'' and ''trans'' configuration, similar to the parent azo-pyridine. The highest occupied orbital with coefficients located at the azopyridine unit is considerably higher in energy for the ''cis'' as compared to the ''trans'' isomer (E<sub>cis</sub>=-6.19 eV(MO=219)), (E<sub>trans</sub>=-6.53 eV(MO=216)). | |||
Version vom 6. August 2009, 12:36 Uhr
Struktur
Orbitale
DOS-Spektrum
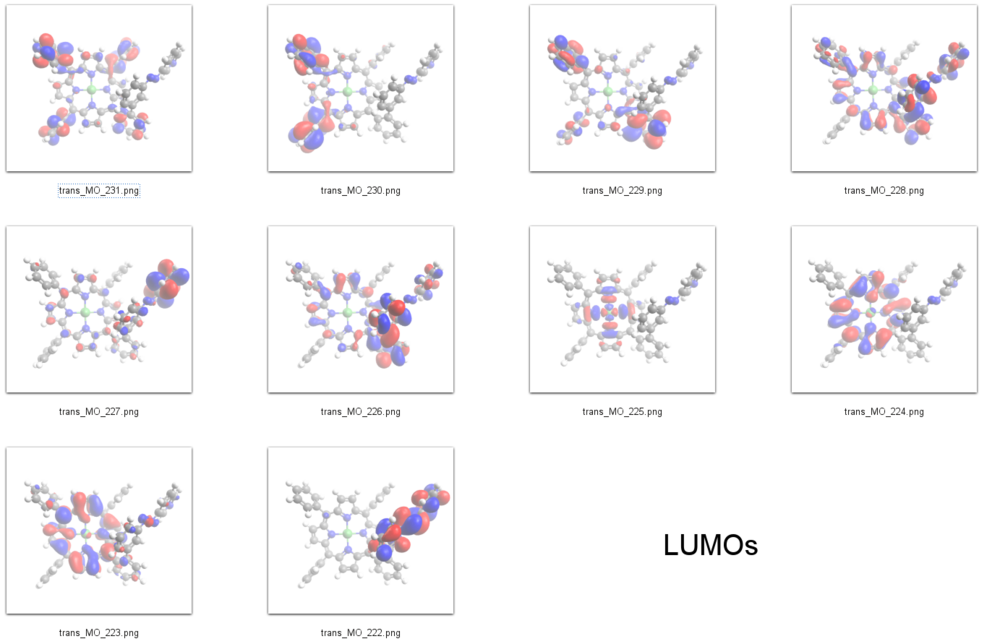
LUMOs
HOMOs
Orbitalenergien
231 L+9 -0.65 A 230 L+8 -0.7 A 229 L+7 -0.74 A 228 L+6 -0.98 A 227 L+5 -1.15 A 226 L+4 -1.17 A 225 L+3 -1.5 A 224 L+2 -2.46 A 223 L+1 -2.49 A 222 LUMO -2.85 A 221 HOMO -5.33 A 220 H-1 -5.46 A 219 H-2 -6.16 A 218 H-3 -6.19 A 217 H-4 -6.39 A 216 H-5 -6.53 A 215 H-6 -6.76 A 214 H-7 -6.79 A 213 H-8 -6.87 A 212 H-9 -6.96 A 211 H-10 -6.99 A
Download
Dateien als Zip-Archiv runterladen
Interpretation of STS-spectra
According to our DFT-calculations (B3LYP/6-311+G*) the Pi-N-Orbitals of the diazapyridine unit differ in the cis and trans configuration, similar to the parent azo-pyridine. The highest occupied orbital with coefficients located at the azopyridine unit is considerably higher in energy for the cis as compared to the trans isomer (Ecis=-6.19 eV(MO=219)), (Etrans=-6.53 eV(MO=216)).