Plattenspieler trans-Orbitale: Unterschied zwischen den Versionen
Zur Navigation springen
Zur Suche springen
| Zeile 52: | Zeile 52: | ||
=== Interpretation of STS-spectra === | === Interpretation of STS-spectra === | ||
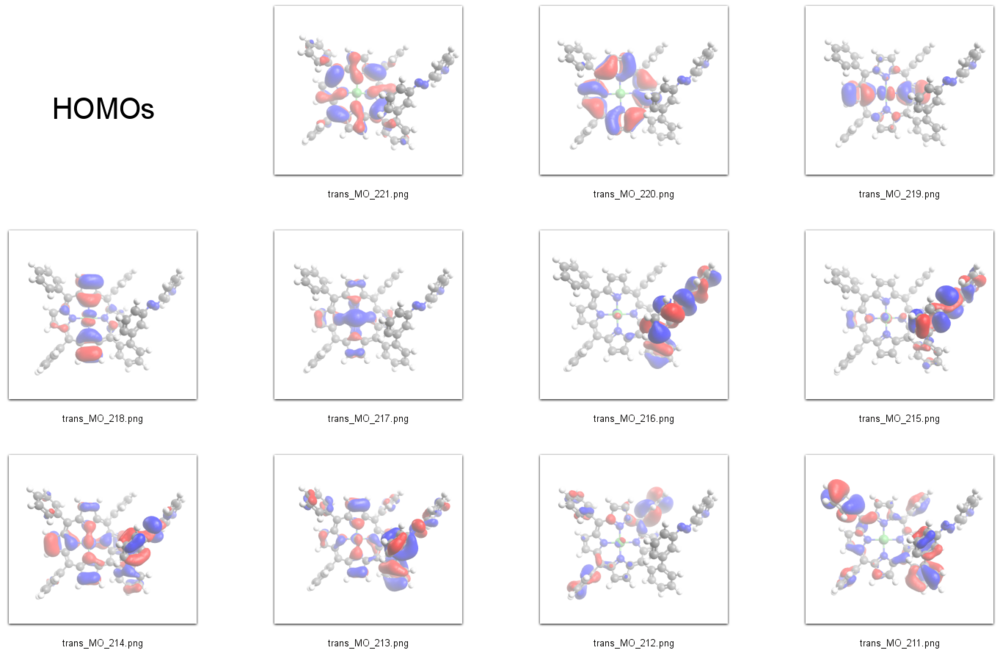
According to our DFT-calculations (B3LYP/6-311+G*) the ''n'' and 𝜋 orbitals of the diazapyridine unit differ in the ''cis'' and ''trans'' configuration, similar to the parent azobenzene. The highest occupied orbital with coefficients located at the azopyridine unit is considerably higher in energy for the ''cis'' as compared to the ''trans'' isomer (E<sub>cis</sub>=-6.19 eV(MO=219)), (E<sub>trans</sub>=-6.53 eV(MO=216)). | According to our DFT-calculations (B3LYP/6-311+G*) the ''n'' and 𝜋 orbitals of the diazapyridine unit differ in the ''cis'' and ''trans'' configuration, similar to the parent azobenzene. The highest occupied orbital with coefficients located at the azopyridine unit is considerably higher in energy for the ''cis'' as compared to the ''trans'' isomer (E<sub>cis</sub>=-6.19 eV(MO=219, n type orbital)), (E<sub>trans</sub>=-6.53 eV(MO=216)). | ||
Version vom 6. August 2009, 12:41 Uhr
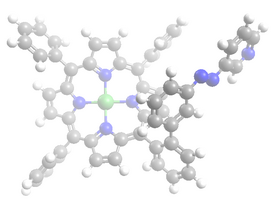
Struktur
Orbitale
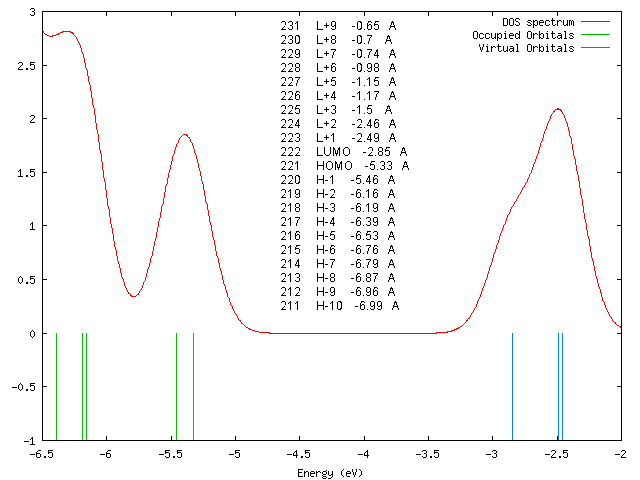
DOS-Spektrum
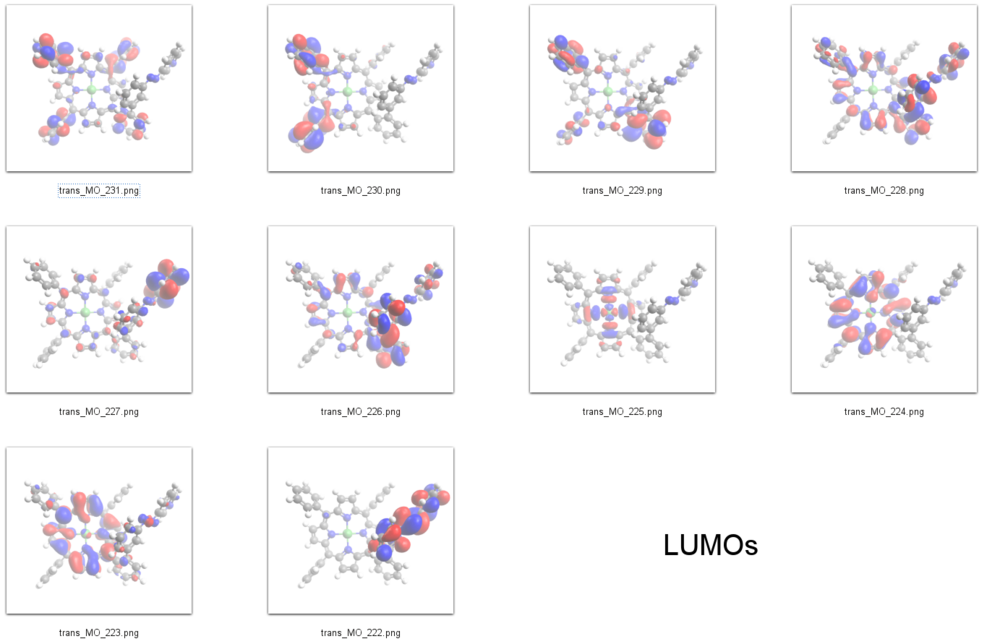
LUMOs
HOMOs
Orbitalenergien
231 L+9 -0.65 A 230 L+8 -0.7 A 229 L+7 -0.74 A 228 L+6 -0.98 A 227 L+5 -1.15 A 226 L+4 -1.17 A 225 L+3 -1.5 A 224 L+2 -2.46 A 223 L+1 -2.49 A 222 LUMO -2.85 A 221 HOMO -5.33 A 220 H-1 -5.46 A 219 H-2 -6.16 A 218 H-3 -6.19 A 217 H-4 -6.39 A 216 H-5 -6.53 A 215 H-6 -6.76 A 214 H-7 -6.79 A 213 H-8 -6.87 A 212 H-9 -6.96 A 211 H-10 -6.99 A
Download
Dateien als Zip-Archiv runterladen
Interpretation of STS-spectra
According to our DFT-calculations (B3LYP/6-311+G*) the n and 𝜋 orbitals of the diazapyridine unit differ in the cis and trans configuration, similar to the parent azobenzene. The highest occupied orbital with coefficients located at the azopyridine unit is considerably higher in energy for the cis as compared to the trans isomer (Ecis=-6.19 eV(MO=219, n type orbital)), (Etrans=-6.53 eV(MO=216)).